Abstract
Introduction
Mesenchymal stem cells (MSCs) isolated from donated tissue have been widely investigated as a treatment for acute graft versus host disease (GvHD), but with mixed results. Factors including MSC donor variability and the effects of prolonged MSC culture expansion may have contributed to inadequate outcomes.
Induced pluripotent stem cells (iPSCs) can proliferate indefinitely without loss of pluripotency. The novel Cymerus™ manufacturing process facilitates a virtually limitless supply of well-defined and consistent MSCs from a single donation. Production is achieved by differentiating iPSCs into MSCs using proprietary clonogenic progenitor-based technology. This avoids both donor to donor variability and the need for excessive culture expansion once MSCs are formed.
We are undertaking a Phase I clinical trial of Cymerus iPSC-derived MSCs (CYP-001) in steroid-resistant acute GvHD (NCT02923375). We believe this will be the first completed clinical trial involving iPSC-derived cells.
Methods
This is a multi-center, open label, dose escalation study to assess the safety, tolerability and efficacy of CYP-001 in adults with grade II-IV steroid-resistant acute GvHD, following allogeneic hematopoietic stem cell transplantation. All subjects had failed to respond to at least three days of steroid treatment (≥1 mg/kg/day), administered in accordance with standard management at each center.
The first eight subjects enrolled in Cohort A received two intravenous (IV) infusions of CYP-001 one week apart, at a dose of 1 x 106 cells/kg, in addition to standard of care medications. After an independent data and safety monitoring board review, the next eight subjects entered Cohort B, in which the MSC cell dose was doubled. Primary evaluation was performed over eight study visits to day 100. Subjects then entered a follow-up phase of up to two years. Data for subjects in Cohort A with a minimum of six months follow-up are presented here.
GvHD was staged and graded according to the 1994 Consensus Conference on Acute GvHD Grading. A Partial Response (PR) was defined as improvement in the severity of GvHD by at least one grade compared to baseline, while a Complete Response (CR) was defined as the absence of any GvHD signs or symptoms. The Overall Response (OR) rate was defined as the proportion of subjects showing either a PR or CR. The primary objective was assessment of the safety and tolerability of two infusions of CYP-001. The secondary objective was efficacy, assessed by best response to treatment, by Day 28 and Day 100 and overall survival at Day 28 and Day 100.
Results
Four males and four females, with an average age of 57 years (range: 45-66) were enrolled in Cohort A during 2017. At baseline, subjects had Grade II (n=3) or Grade III (n=5) steroid-resistant acute GvHD. One subject had skin, gastrointestinal (GI) and liver involvement; four subjects had skin and GI involvement; two subjects had GI involvement only; and one subject had skin involvement only.
The treatment was well tolerated in all cases, and there were no treatment-related Serious Adverse Events (SAEs) reported. Three subjects experienced SAEs that were not considered to be study drug related: (i) febrile neutropenia, hypokalemia and parainfluenza, each of which resolved; (ii) a lower respiratory tract infection, which resolved; (iii) pneumonia, which was fatal.
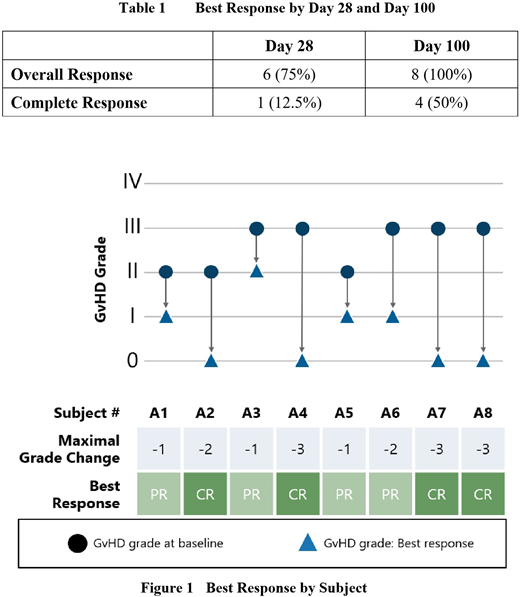
All eight subjects showed at least a PR. Four subjects achieved a CR by Day 100. In all four cases where a CR was achieved, it was then sustained until Day 100. The median GvHD grade at Day 100 was 0 (range: 0-II), compared to a median grade of III (range: II-III) at baseline. Disease progression (an increase in the severity of GvHD by at least one grade compared to baseline) was not observed in any subject at any study visit. Overall survival was 7/8 (87.5%) six months after the first infusion of CYP-001.
The best response rates by Day 28 and Day 100 are summarized in Table 1, while the maximal response by individual subject is illustrated in Figure 1.
Conclusion
Infusion of CYP-001 at 1 x 106 iPSC-derived MSCs/kg was safe and well tolerated in this patient cohort. Treatment response and overall survival rates are encouraging compared to previously published outcomes. The Cohort B primary evaluation period is expected to be completed by September 2018, and progression to a Phase II trial in this clinically challenging disease will then be considered.
Bloor:AbbVie: Research Funding; Janssen: Research Funding. Radia:Mallinckrodt: Research Funding. Yeung:Novartis: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Pfizer: Honoraria; Amgen: Honoraria; Specialised Therapeutics Australia: Honoraria. Slukvin:Cynata Therapeutics Limited: Consultancy, Equity Ownership. Kelly:Cynata Therapeutics Limited: Employment, Equity Ownership. Rasko:Gilead: Honoraria; Abbvie: Speakers Bureau; Takeda: Speakers Bureau; International Society for Cellular Therapy: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Speakers Bureau; Cynata: Consultancy, Honoraria; bluebird bio: Honoraria, Other: Clinical trials ; Spark: Consultancy; FSHD Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Current Cure The Future Foundation: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria; Pfizer: Honoraria; GSK: Honoraria; Genea: Equity Ownership; IMAGO Biosciences: Consultancy; Rarecyte: Consultancy, Equity Ownership; Gene Technology Technical Advisory, OGTR, Australian Government: Other: Chair; Advisory Committee on Biologics, Therapeutics Goods Administration, Australian Government: Other: Past Chair.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal